Terumo Cardiovascular: MEDUSA4 at the Pulse of Product Assembly
“MEDUSA4 has dramatically improved our process for designing and controlling our manufacturing specifications. This improvement has benefited our customers by decreasing turnaround time for implementation of new and revised designs. The customer support that the MEDUSA4 programmers have provided has been exceptional. We can really count on getting quality support in a timely manner.”
– Bob Capistran, Drawing and Design Manager at Terumo CVS in Ashland, MA, USA
When it comes to matters of the heart, surgeons around the world depend on the U.S.-based specialist company Terumo Cardiovascular Systems Corporation (Terumo CVS) to provide leading-edge technology for patient care. Terumo’s product design and drafting department in Ashland, Massachusetts, focuses on a line of essential disposable products for cardiac surgery. Here, CAD Schroer’s MEDUSA4 DRAFTING and diagramming package and the MEDINFO document management system are at the pulse of the company’s engineering design and data management processes.

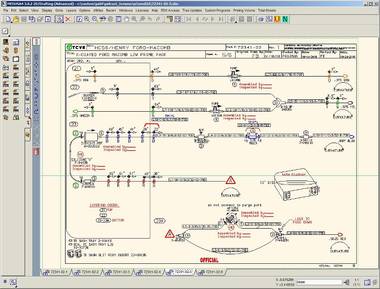
About Terumo Cardiovascular Systems Corporation
Custom Assembly Process Drawings
At Terumo CVS in Ashland, every device assembly drawing is a custom drawing based on individual order specifications from a cardiac surgery center. The company has an extensive library of custom symbols for the parts it uses in assembling its boxes of disposable medical devices. These devices are used by expert surgeons around the world and include state-of-the-art self-venting blood oxygenators.
Bob Capistran is the Drawing and Design Manager at Terumo CVS. “MEDUSA4 has dramatically improved our process for designing and controlling our manufacturing specifications,” he says. “This improvement has benefited our customers by decreasing turnaround time for implementation of new and revised designs.”
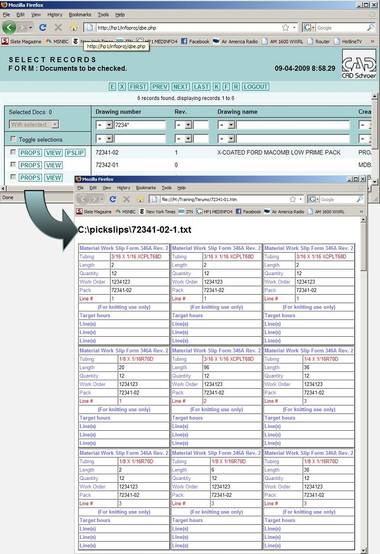
MEDInfo™ – Flexible Web-based Data Management
Customizations for Process Optimization
Other MEDInfo customizations include:
- Allowing only the latest revision of a design to be viewed/printed by “read-only” authorized users
- Drawing locking and notification if a new revision is in-work by the drafting department
- Auto-generation of multi-page PDF documents of all approved designs
Multi-sheet management of drawings
ERP Systems Integration
After design release, the system automatically generates bills of materials, which are sent to Terumo CVS’ materials management system for purchasing and production
“MEDUSA4’s flexibility and customizability allows for easy integration into existing corporate processes and systems,” explains Don Terepka, Technical Consultant at CAD Schroer’s U.S. office in New York. “Each company is unique, and we work together with clients to create and support custom tools that optimize their design, development and production processes.”
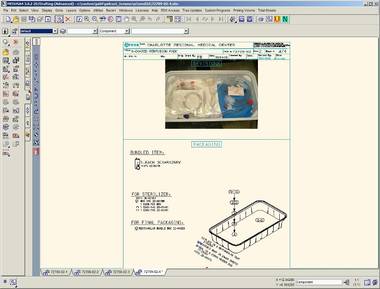
Obsolete Parts Management
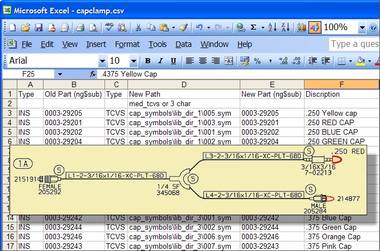
Another example of a customization for significant efficiency gains is an Obsolete Parts Management Tool developed by CAD Schroer for Terumo CVS. As technology moves on and parts or materials for medical devices change, Terumo CVS needs to update its designs to reflect such changes. This has to be done efficiently, limiting the amount of costly and error-prone manual interventions required.
CAD Schroer devised a solution which accesses a central spreadsheet that tracks all existing and replacement parts.On demand, all drawings in the database can be updated with new parts as mapped by the spreadsheet. PDF copies of all graphically modified drawings are then automatically regenerated for Web-based view-only use.
Fast and Reliable Support
Under its maintenance agreement, CAD Schroer offers full support for the customizations it writes for clients.
Terumo CVS’ Bob Capistran works in partnership with CAD Schroer’s technical consultants. “The customer support that the MEDUSA4 programmers have provided has been exceptional. We can really count on getting quality support in a timely manner,” he concludes.
For more information about Terumo Cardiovascular Systems, please visit www.terumo-cvs.com
Terumo is a registered mark of Terumo Corporation, Tokyo, Japan.

